Screening Cyclic Peptides With Hydropore
By Indee Labs
The process of delivering molecules into the interior of cells, known as intracellular delivery (ICD), is highly sought after by scientists. Like anything worth it, however, it’s quite challenging.
Luckily, recent achievements in microfluidic techniques have introduced new mechanoporation (using mechanical energy) approaches that can effectively deliver things into cells. However, these approaches aren’t yet the Holy Grail we’ve been looking for.
How Does Hydropore Deliver Cyclic Peptides?
Cyclic peptides can target specific interactions between proteins inside of cells, something that’s quite challenging with drugs under normal circumstances. Unfortunately, these peptides typically can’t enter cells on their own. Without intervention, studying their functionality is difficult at best.
Microfluidic-based mechanoporation, like Hydropore, temporarily renders cells permeable, meaning molecules can temporarily enter the cells by applying hydrodynamic forces to the cell membrane. It’s a fluid shearing-based technique that’s successfully delivered nucleic acids and gene editing complexes into human cells by using an oscillating motion to disrupt the lipid membrane.
Hydropore is based on microfluidic vortex shedding. Essentially, microfluidic vortex shedding (μVS) enables scientists to deliver constructs to cells with hydrodynamic forces. An external team then went one step further with dispensing-microfluidic vortex shedding to improve the experimental throughput of the platform with SBS-format well plates.
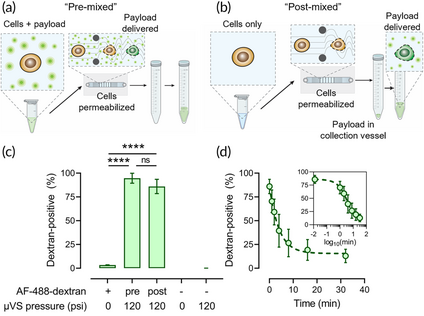
Figure 1 A short window of cell permeability after Hydropore-processing.
Dispensing Microfluidic Vortex Shedding with Hydropore
Said team improved on current μVS methodologies by integrating Indee’s μVS chip in a system called dispensing-microfluidic vortex shedding (DμVS). They started by timing how long cells remain permeable after processing. Equivalent delivery efficiency (ie. 80~90%) was achieved for pre- and post-mixed cells within a few minutes of Hydropore processing.
They aimed to create a system to perform vector-free ICD on a larger scale. This new system rapidly moves permeabilized cells to standard microplates with different concentrations of peptides to speed things along and streamline the process.
They added four new components to Hydropore:
· An electronic pressure regulator
· A flow sensor
· A microsolenoid valve-based dispense tip
· An x-y motor stage
DμVS enabled efficient and precise delivery of permeabilized cells to multiple wells on microplates, improving the throughput and consistency of their experiments.
Figure 2 Overview of the DµVS system.
Test Results
The team tested the DμVS system using cyclic peptides and demonstrated that it could enhance the delivery of these peptides into cells. The system handled large numbers of samples in a high-throughput manner, enabling efficient screening of these cyclic peptides in different cell-based assays.
Is Generalization Possible?
After finding that DμVS successfully delivered their chosen peptides into the cell, the team wanted to know if their technique would be effective with other peptides. They tested an additional 15 peptides and found that over nine didn’t show any cellular activity, despite several of them binding successfully to MDM2.
Figure 3 Screening of additional peptides.
Using the DμVS system, they characterized a set of peptides with varying binding and activity levels. They found that some peptides showed improved activity after being delivered using DμVS, while others showed no activity despite their strong binding affinity. This information can help guide further optimization and understanding of these peptides by allowing for hundreds of experiments in minutes. Thus, allowing scientists to quickly select promising cyclic peptides based on their biological activity prior to downstream drug development.
Future Directions
While others are using Hydropore in cell engineering for cell therapy, this team saw it as a way to better understand target engagement in cell-impermeable molecules. The DµVS system allows scientist to de-risk cyclic peptide drug development by screening candidates based on biological activity before the laborious process of making peptides cell permeable.
There are many potential areas of further investigation, such as understanding the properties that affect cell permeability and payload concentration and exploring the upper limits of payload size that can be delivered using DμVS.
In Conclusion
Overall, the DμVS system offers a promising approach for delivering substances into cells and studying their effects, particularly for cyclic peptides targeting challenging intracellular proteins. This system can hopefully contribute to the toolkit of technologies used in drug discovery.